Homopolymers, Copolymers and Polymer Blends (Alloys).
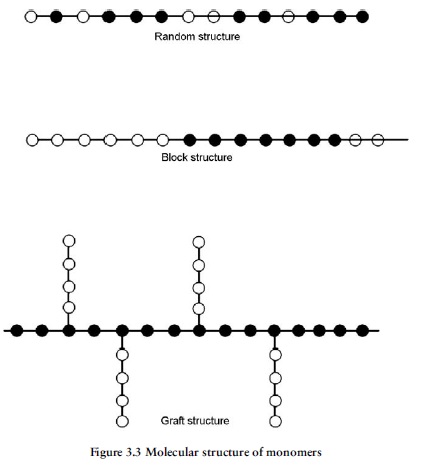
When polymerisation a single monomer type (e.g., ethylene or styrene) it polymerised to produce a polymer (polyethylene or polystyrene) in which the molecular chain was made up of the same repeat unit throughout its length. Polymers of this type are known as homopolymers.It is possible, however, to produce polymers from a combination of two or more monomers, the final molecule chain then having a combination of repeat units corresponding to the monomers selected, rather like a necklace with different coloured beads along its length. This type of polymer is known as a copolymer. Examples of copolymers include SAN (e.g., Tyril from Dow chemicals), a copolymer based on styrene and acrylonitrile as monomers. ABS is a rather complex copolymer based upon three monomers, acrylonitrile, butadiene and styrene. Another widely used copolymer is based upon propylene and ethylene monomers and is usually called propylene copolymer, as distinct from polypropylene, which is the homopolymer based
on propylene alone.
These copolymers have been developed mainly in order to overcome the specific shortcomings of the related homopolymers. For example, SAN is a much more heat- and solvent-resistant material than the homopolymer polystyrene. ABS has similar advantages over polystyrene, plus the additional benefit of greater toughness due to the butadiene content. With propylene copolymer, the presence of the ethylene-derived units in the polymer chain overcomes to a considerable extent the brittleness at subzero temperatures displayed by the homopolymer polypropylene.
The properties of individual copolymers can also be varied by altering the relative proportions of the monomers involved, or by arranging them in different patterns along the length of the molecular chain.
The development of polymer blends, or alloys, has further expanded the range of materials for injection and other moulding processes. With this type of material, two or more polymers are produced separately and then blended or ‘alloyed’ together to form
new materials having a combination of properties not possessed by the constituent polymers individually.
Examples of polymer blends include Noryl, from General Electric, a blend of polystyrene and polyphenylene oxide, or Bayblend, a combination of ABS and polycarbonate produced by Bayer.