Thermoplastics can be subdivided into two distinct classes based upon differences in their molecular structure.These differences can have a significant bearing on the performance of mouldings in service, and have a marked effect on the behaviour of the material
during processing.
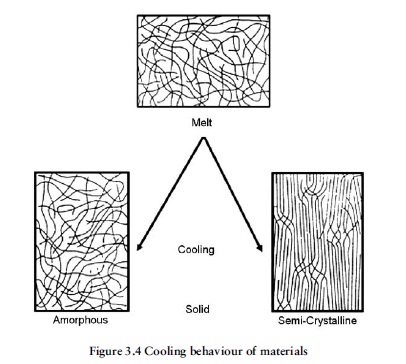
Materials such as polystyrene, polycarbonate, acrylics, SAN, ABS and PVC are said to be amorphous thermoplastics. This signifies that in the solid state their molecular structure is random and nonordered, the long-chain molecules being all entangled rather like
solidified strands of spaghetti.
Materials such as most of the Nylons, acetal, polypropylene, polythene and the thermoplastic polyesters, have a much more ordered structure in the solid state than amorphous materials. They have a considerable proportion of the long-chain molecules
packed closely together in regular alignment. These materials are known as semicrystalline thermoplastics. It should be noted, however, that with both types of material the molecular structure is amorphous at melt temperature.
Most amorphous thermoplastics are transparent in their natural, unpigmented form, although ABS, for example, is an exception, while most semicrystalline thermoplastics in their solid unpigmented form are translucent or an opaque white colour. It is interesting to observe (for example when ‘purging’ on injection moulding machines) that fully molten natural polypropylene or acetal are initially transparent, but as the melt cools it clouds over, becoming translucent in the case of polypropylene and opaque white in the case of acetal. This clouding is due to the material’s molecular structure gradually rearranging itself from the tangled amorphous state in the melt to the more ordered semicrystalline state in the solid.
Melting and Solidification
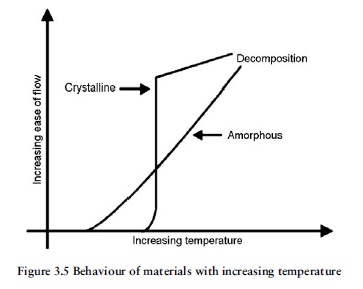
Amorphous thermoplastics exhibit a progressive softening over a wide temperature span, whereas the semicrystalline materials rapidly change from the solid melt condition over a quite narrow temperature band. Conversely, when amorphous materials are cooled they solidify slowly over a wide range of temperature, in contrast to the semicrystalline plastics, which change from melt to
solid over a narrow range of temperature.
Shrinkage
Amorphous thermoplastics display very low shrinkage when they solidify, typically between 0.5% and 1%. Semicrystalline materials shrink very much more, usually between 1.5% and 5%depending upon the particular material.
The higher shrinkage with the semicrystalline materials is due to the repeat units along the molecular chains being of such a form that they can pack very closely together in an ordered manner depending on the moulding conditions being used. For example, when semicrystalline thermoplastics are moulded in hot moulds, cooling
rates are slow to allow more time for the molecular chains to disentangle themselves and take up their crystalline structure. This results in a greater proportion of the material being in its crystalline state (higher crystallinity), giving a product with superior mechanical strength and dimensional stability, but with relatively high shrinkage.
If the same material is moulded in a cold mould, the more rapid cooling will inhibit the formation of crystalline areas. The resulting lower level of crystallinity will give the product inferior physical properties, and lower shrinkage accompanied by a tendency for
dimensional instability and distortion during later service due to continued aftermoulding shrinkage.